Abstract
Background: Multiple Myeloma (MM) accounts for roughly 10% of all hematologic cancers in the U.S., with 37% of MM patients being non-white minorities. Advanced stage at diagnosis and specific cytogenetic abnormalities are associated with high-risk disease and poor prognosis. Studies evaluating these risk factors, however, consisted of mostly Caucasian patients with an underrepresentation of ethnic minorities. In the phase III DETERMINATION trial, ~18% of the study population was Black compared to only ~6% Hispanic, with the rest being Caucasian. Although this study showed significant overall progression free survival (PFS) benefit in transplant patients, this was not seen in the Black patient subgroup. To further investigate outcomes of other ethnic MM patients in the Central Valley of CA, we analyzed characteristics and outcomes of MM patients who were seen at Community Medical Centers in Fresno CA (49.6% Hispanic population).
Methods: Patients diagnosed with MM at Community Medical Centers from January 2011 to December 2020 were included. High risk disease was defined by the Mayo Clinic mSMART 3.0 classification. Demographic and disease-relevant information including age at diagnosis, gender, ethnicity (Hispanic vs non-Hispanic), insurance, median income by zip code, laboratory data at diagnosis (hemoglobin, creatinine, corrected Ca, LDH, beta 2 microglobulin), immunoglobulin type, light chain type, cytogenetic abnormalities, stage (R-ISS), 1st line treatment (doublet vs triplet), transplant status, and treatment response (IMWG response criteria) were collected. Comparisons between Hispanics and non-Hispanics were made by Fisher's exact test and nonparametric equality-of-medians test where appropriate. Outcomes were compared via log-rank testing and Kaplan-Meier estimates for 2 year PFS and overall survival (OS). Univariate cox proportional hazard model for survival was used to examine the association of demographic and MM variables with mortality risk after first line therapy.
Results:
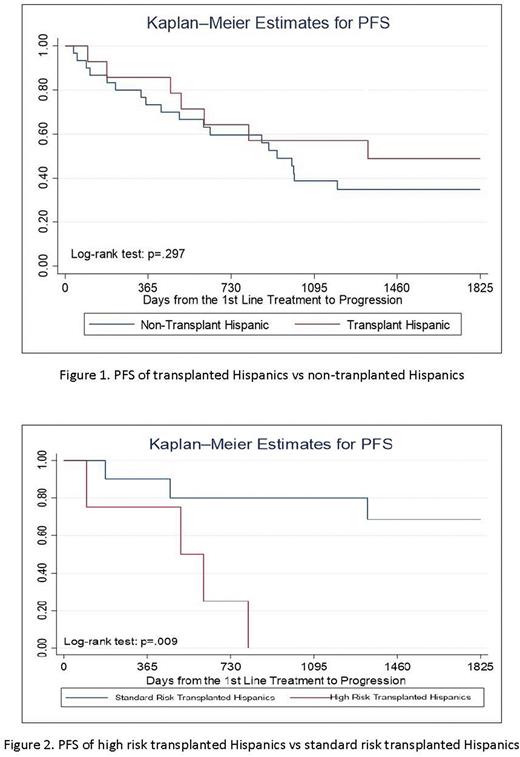
117 MM patients were included with 42 high risk patients identified (11 Hispanic, 31 non-Hispanic). Median age at diagnosis of Hispanics vs non-Hispanics was 61.7 vs 66.5 years (p=0.046). Hispanic vs non-Hispanic median income was $49734.50 vs $62588.90 (p=0.003). Insurance status differed significantly with more Hispanics having Medi-Cal and non-Hispanics having private/Medicare (p=0.001). 13 non-Hispanics had 17p del vs 0 Hispanics (p=0.001). Hispanics and non-Hispanics did not have significantly different responses to first line therapy per IMWG criteria (p=0.535). Hispanic and non-Hispanic patients experienced similar 2 year PFS and OS (PFS: 63% vs. 60%, p=0.744; OS: 94% vs 85%, p=0.145). 14 Hispanics and 20 non-Hispanics underwent autologous stem cell transplant. No significant difference in 2 year PFS was seen in transplanted Hispanics vs transplanted non-Hispanics (p=0.228) and transplanted Hispanics vs non-transplant Hispanics (p=0.297). High risk transplanted Hispanics showed significantly worse 2 year PFS vs standard risk transplanted Hispanics (25% vs 80%, p=0.009). Transplanted Hispanics differed from non-transplant Hispanics by median age (55.5 vs 67, p=0.05) and income ($56510.00 vs $42418.00, p=0.01).
Conclusions: Hispanic MM patients were younger at diagnosis, earned a lower median income, and had more Medicaid coverage compared to non-Hispanics. These differences did not translate to a difference in first line therapy, treatment response, or survival. Transplanted Hispanics were younger and had higher income than non-transplant Hispanics, suggesting that socioeconomic factors may play a role in transplant eligibility. Undergoing transplant, however, did not show a significant PFS benefit compared to Hispanics and non-Hispanics who did not undergo transplant. High risk Hispanics still had inferior PFS compared to standard risk Hispanics after transplant. Additional studies and longer follow-up are needed to confirm these findings. If validated, they could potentially influence treatment decisions for Hispanic MM patients.
Disclosures
Bukari:SANOFI: Honoraria; NeoGenomics Laboratories, Inc: Research Funding; EUSA Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics, Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; HCG-MorphoSys: Honoraria, Membership on an entity's Board of Directors or advisory committees. Abdulhaq:Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jansen: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Speakers Bureau; Alexion: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal